
| |

|
earthDr! "the
environmental ombudsman"
earthDRx.org - prescriptions (Rx) for |
|
Importance in Determining whether Gaseous Diffusion or Bulk Flow is Responsible
for Vapor Intrusion into Structures
|

|
In Figure 1, a wetting front gravity drains due to the infiltration and then the percolation of a recent rainfall event. As a wetting front develops and vertically drains into the underlying subsoil, the soil atmosphere now trapped below the wetting front and above the underlying water table is compressed into a smaller volume resulting in an increase in the soil atmospheric pressure. This increased pressure can then be the driving force necessary to push soil air through the foundation wall and/or the basement slab of a dwelling. The greatest increase in soil atmospheric pressure of which I have knowledge is an 18-inch water pressure increase during the development of one such wetting front as this water percolated to the water table.
Even less dramatic soil atmospheric pressure increases can be much more than a mild concern. This is since, a not uncommon regulatory value prescribed to demonstrate (what the regulators call: commissioning) that a vapor intrusion remedial system installed at a home or business is effective requires an expressed vacuumm of a mere 0.004-inches (measured as: inches of water column) or greater water pressure decline immediately below the affected basement slab. I must question if a radon-type fan is capable of achieving the necessary vacuum, under the limited range of vacuums, that can be expressed to prevent vapor intrusion from the drainage of such a significant wetting front where subsurface climatic conditions causes bulk flow to predominate. When gaseous diffusion predominates, as the mechanism for subsurface transport of the soil air, a decrease of at least 0.004-inches of water pressure can be often be maintained by a radon-type fan, but it is questionable that a decrease of 0.004-inches of water column can be attained when bulk flow predominates. Unfortunately, in regard to the monitoring of these remedial systems, demonstration of performance were most often done on larger-diameter vertical runs of piping, and not horizontal runs of piping, to determine flow rates. I would argue that these flow rates are over reported due to vertical cycling. Flow rates must be measured in horizontal runs of piping at a point that is a minimum of 5-pipe diameters away from any coupling.
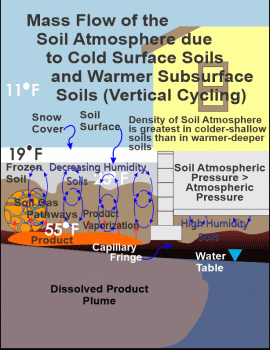
During the wintertime, Figure 2 also illustrates bulk airflow through the soil atmosphere. Please note that the soil surface is depicted as sealed off by a snow cap and the underlying frozen shallow soils. In this example a temperature differential results in pressure differences driving the lower-density warmer soil air
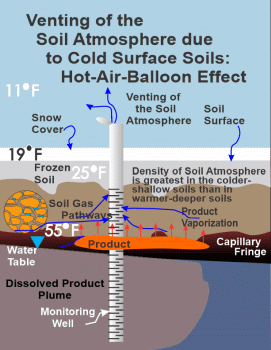
|
Figure 3 is used to illustrate wintertime conditions. Under unsaturated flow conditions, soil moisture tends to move from warmer to colder soils (when all things being equal like soil texture and soil structure are homogeneous): most often as water vapor; and, to a lesser extent as liquid water moving as
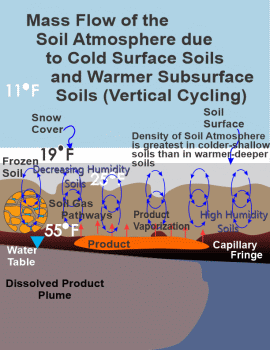
|
Figure 4 illustrates when gaseous diffusion dominates. This figure can be indicative of conditions in mid to late spring into mid to late autumn in a temperate climate. A surface cover of asphalt, as opposed to grass, is used to illustrate more extreme surface and subsurface temperature conditions. It always struck me that when excavations at gasoline stations initially broke through asphalt, overwhelming odors of gasoline were noted when it was between mid to late spring til mid to late autumn.
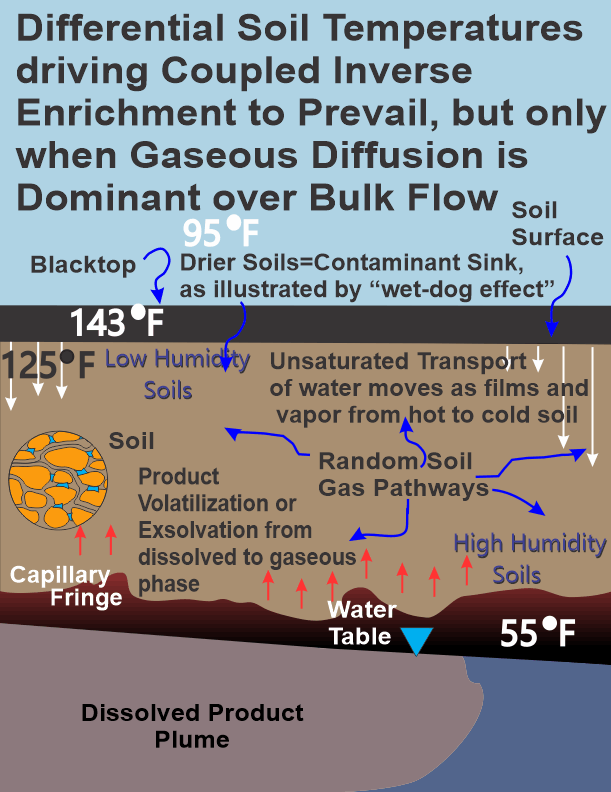
|
What can explain why there are such overwhelming odors when pavement is broken at a gasoline station? When a dog is bathed there is such a strong repugnant odor. The volatile organics in the dog's fur unsuccessfully compete with water for the bonding sites present on the fur as it is wetted and, then, these repugnant smelling volatiles off gas from the fur as water replaces the volatiles on the fur's bonding sites. This wet-dog effect is like the wet-soil effect: volatile organic sorption to soil is reduced as water sorption to the soil increases. Conversely, more sorption of volatile organics to the soil is possible as it dries. Just to emphasize, as the moisture content in a soil horizon increases, it will desorb any previously sorbed volatile organics. Volatile enrichment in dry soil followed by desorption of volatile organics as it is wetted; and, then displacement of the soil atmosphere results in bulk flow and transport of contaminants into structures.
|
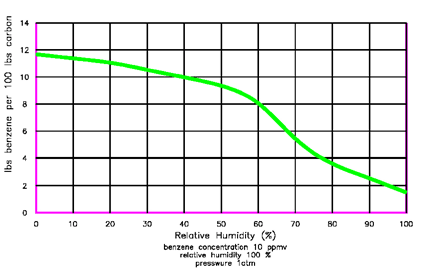
Figure 5 illustrates competition for bonding sites between water and benzene on vapor-phase carbon. This figure indicates that as the water vapor concentrations in an air stream increase less benzene in an air stream can be sorbed by vapor-phase carbon. This actual example of carbon sorption indicates that which can also happen in the soil.
Back to Figure 4, note that in the heat of a hot summer, the highest density soil air is at depth in the deeper soils and the least density soil air is in the shallow soils; soil air density is mostly due to soil temperature and soil humidity. Shallow soil temperatures are now much higher than in the deeper subsurface soils as opposed to winter-time conditions; therefore, increased soil air density with depth is not a driving force for bulk flow now that deeper soils are colder than shallow soils. If a soil is completely homogeneous, then for a good approximation, unsaturated flow of water goes from hotter to colder soils both as water vapor; and, as films of liquid-phase water from one soil particle to an adjacent soil particle. In this situation water is moving vertically downwards as unsaturated water flow as films of water partially driven by temperature gradients; and, as water vapor moving in all directions by gaseous diffusion.
|
Gaseous diffusion is just the random movement of gas molecules as illustrated in this fourth figure. Gas molecules will move in any direction in the soil pores unless its path is blocked by local occlusion of water, soil structure (including cutans = clay skins. Clay lining of larger soil pores often occurs and is known as cutans or clay skins), or soil biota. Both the volatile contaminants plus natural gaseous species present in the soil atmosphere such as nitrogen, oxygen, carbon dioxide, water vapor, and methane will randomly move in all directions.
The volatile organics will preferentially sorb to the driest soil (the hot shallow soils depicted in the fourth figure) and the water vapor will preferentially sorb to the colder soils (depicted in the fourth figure). The warmer, therefore, drier soils will act as a trap for the randomly moving volatile organics present in the soil atmosphere, as volatile organics contact these drier soils they sorb to the soil particulates and are removed from ongoing random gaseous movement. Continued random motion of any volatile organics that remain in the soil atmosphere will eventually randomly move to these drier soils, repetitively allowing more and more molecules to be trapped by these drier soils. This continued trapping of volatile contaminants by sorption to the soil particles is not unlimited. At some point when sorption (bonding sites are full) is exhausted, any volatile organic contaminant straying into these drier soils will randomly move through and eventually out of these drier soils or should they be sorbed to the soil they will displace some other volatile from the soil; thereby, not exceeding the maximum sorptive capacity of the local soil. The volatile organics will enrich in the drier, hotter soils; and, water will enrich in the colder soils. I have coined the phrase for this phenomenon: "Coupled-Inverse-Enrichment" where water is increased in the deeper soils and volatiles are increased in the shallow soils due to soil temperature differentials particularly during those periods of drier-hot summer months. This is the mechanism for enrichment of volatile contaminants below these very dry soils under the pavement at a gasoline station.
Now let us stay with the condition that gaseous diffusion is defining soil air flow. Assume that the pavement is replaced with grass; that volatile organic enrichment of surface soils has occurred near a home during a somewhat prolonged hot and droughty period (say 2 weeks) in the summer; and, followed by a fairly significant rainfall event with subsequent gravity drainage of a wetting front (illustrated by the first figure). These
|
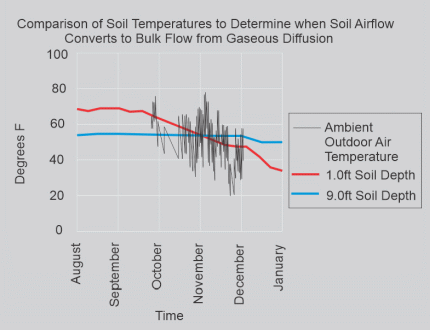
The significance of volatile soil enrichment and then vapor intrusion into structures is of great import when soil air flow switches from gaseous diffusion to bulk flow which is due to soil temperature change from warmer surficial temperatures of summer to colder surficial temperatures in autumn. In my locale, conversion from gaseous diffusion to bulk flow is typically in the autumn with bulk flow lasting through the winter and into the spring when it eventually converts back to gaseous diffusion. The wide swing in the ambient temperatures, illustrated in Figure 7, is due to being recorded twice per day during the daytime and the nighttime. Monitoring soil temperatures over time while comparing both shallow (~1-foot depth) and deep (~9-foot depth) temperature plots over time identifies when gaseous diffusion converts to bulk flow as displayed in Figure 7 on this page. Note that the shallow soil (~1-foot depth) temperature decreases as the autumn progresses and that the deeper soil (~9-foot depth) temperature remains relatively constant.
At that point in time when the 1-foot soil temperature plot intersects the 9-foot soil temperature plot indicates that soil airflow converts to bulk flow from gaseous diffusion.
|
But why is there such high volatile concentrations available for vapor intrusion into structures? The combination of the elevated volatile organic concentrations locally present at depth in the soil atmosphere, which originates from the maximum zone of contaminant mass in the soil (this source recharge zone can be though as a continous stream of contaminant mass available for offgassing to the soil atmosphere) plus votalites from the formerly contaminant enriched shallow soil atmosphere due to volatile enriched warmer, necessarily drier, shallow soils, provided these soils have continued to remain drier having had no recent precipitation events, promotes the measured spike in vapor intrusion.
The timeframe when the worst case volatile organic concentrations in a home basement is often identified by the initial conversion from gaseous diffusion to bulk flow whether it occurs in the fall or later in the winter. Remember Figure 2 illustrates the situation where the warmer deeper, less-dense contaminated soil air vents into a monitoring well. This logic can be applied to explain that this mechanism is also responsible for the transport of volatile organic contamination into the basement of a structure when the conversion from gaseous diffusion to bulk flow occurs since the basement air pressure is often, if not always, less than the soil atmospheric pressure. I have often measured higher volatile concentrations entering through the basement wall than through the basement slab. The proper operation of a home or business furnace requires the introduction of basement dilution air to ensure the proper combustion of gases. This dilution air further reduces the air pressure in the basement. The lower pressure of the basement air relative to the soil atmosphere promotes vapor intrusion from the soil atmosphere surrounding a basement.
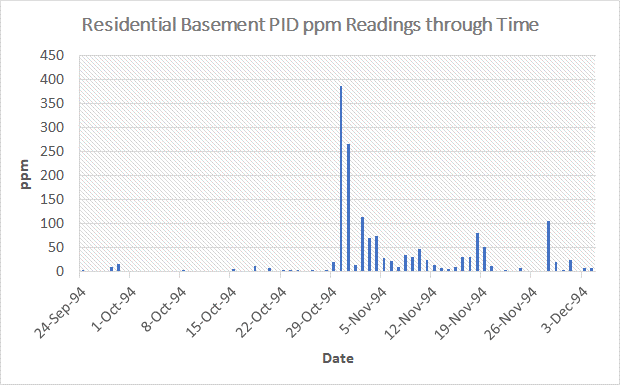
Again, the third and sixth figures depicts the vertical-cycling mechanism for bulk flow during the wintertime. Please note that these figures are indicative of sometime after the initial conversion from gaseous diffusion to bulk flow, as indicated by the frozen shallow soils. In Figure 8, the late stage plot of the volatile organic data measured by a PID meter, sometime after the flip from gaseous diffusion to bulk flow, illustrates the continued intrusion of the soil atmosphere into a home, but at much reduced intrusions relative to that point in time when airflow in the soil atmosphere first flips from gaseous diffusion to bulk flow. Remember that the initially exaggerated soil atmospheric vapor concentrations entering a home are likely the result of the combination of possibly a quite elevated shallow soil concentration, due to the aforementioned enrichment from "Coupled-Inverse Enrichment," in addition to the deeper elevated volatile concentrations of the soil atmosphere near the source zone. These deeper elevated concentrations are now diluted by spatially distant less and less contaminated soil air to clean soil air, resulting in less contaminated vapor intrusion, particularly once the shallow soils have been exhausted of the trapped volatile organics. Where subsurface contamination exists, the things to look for are a spike in volatile contaminant concentrations in basements when soil airflow converts from gaseous diffusion to bulk flow and thereafter, elevated but lesser concentrations until airflow converts back to gaseous diffusion in the springtime. The preceding also applies to homes and businesses with a slab on grade, and not just to basements.
CONTINUATION OF VAPOR REMEDIES FOR DISSOLVED PRODUCT SEEPS
VAPORS OFF GASSING FROM SEEPS OF CONTAMINATED GROUND WATER
TREATISE - REMEDIATION OF VAPORS RESULTING FROM PRODUCT SEEPS